IMPORTANT INFORMATION
1. Introduction

Joan of Arc at the Coronation of Charles VII (1854), Jean Auguste Dominque Ingres.
Joan of Arc (1412-31) was an historical foid who lived during the Hundred Years’ War, a great conflict between France and England.
Born in the peaceful French village of Domrémy, Joan suffered from schizophrenic delusions since early childhood. At the age 17, she hallucinated that an evil spirit was instructing her to lead France’s army to victory over the English. She was so insistent about this that she was granted an audience with the royal court. Upon hearing her prophecy, the imbecilic King of France, Dauphin Charles VII, took her at her word and placed her in command of a battalion of soldiers.
Joan’s first military campaigns were successful. In 1429, Joan and her soldiers relieved the besieged French at the Siege of Orleans, earning her the title of “The Maid of Orleans.” In May, 1430, however, her fortunes changed dramatically and she was routed and captured by the English in Compiègne. After a short trial, she was sentenced to death by burning at for the crime of witchcraft.
Thousands of people witnessed Joan’s execution, and many accounts of her death survive to this day. As she burned at the stake, the following signs were observed:
1. Her muscles contracted and tensed up.
2. Her breasts became full and began to heave violently.
3. She cast her eyes up to heaven, and her expression was not one of pain, but something approaching spiritual ecstasy.
4, In the instant before she lost consciousness, Joan moaned out Jesus’s name three times, loud enough to be heard over the roar of the flames.
5. Joan’s vagina, in contrast to the dry kindling that burned around her, became lubricated and wet.

Individually, each of these signs do not point to a clear conclusion. But taken together, they indicate an undeniable certainty: as the flames enfolded and consumed her body, Joan of Arc was caught within the grips of a powerful orgasm.
2. Masochism
Masochism is defined as “the condition of experiencing recurring and intense sexual arousal in response to enduring moderate or extreme pain, suffering, or humiliation.” It’s described as an addiction-like tendency, with features resembling drug addiction: craving, intoxication, tolerance, and withdrawal.
Masochism is seen as aberrant in males and treated as a harmful psychological disorder.
Female masochism, on the other hand, does not suffer from the same stigma, given its ubiquity.
Confirming this, In Three Essays on the Theory of Sexuality (1924), Sigmund Freud described the three essential traits of femininity -- narcissism, passivity, and masochism. To Freud, pain was an inseparable part of the intensity of a woman’s sexual pleasure, “an expression of the feminine being nature.” He found in women a persistent need for punishment and humiliation, "which succeed [...] in binding erotically the destructive trends which have been diverted inwards."
There are numerous examples of masochism in females:
A. Women overwhelmingly prefer larger-than-average penises.
Here are some quotes from a VICE article:

 www.vice.com
www.vice.com
The top selling dildos on the internet are eight to nine inches long. In the absence of a large cock, some women resort to bestiality with dogs and horses, animals with large cocks.
However, a penis of this size inflicts injuries and perforations on the vagina and causes lasting damage in the form of tears in the cervix, as well as dilation of the vaginal opening. Infections can occur, and there are several case reports of pelvic abscess and subsequent scarring due to insertion of large objects into the vagina.
B. Women love anal sex, despite the lack of an erogenous zone there.
Homosexual men also engage in anal sex. But keep in mind that gay men have erogenous zones deep within their ani known as prostate glands. Women lack this, and so in theory anal sex should be not pleasurable for them, and instead extremely painful. Nevertheless, women engage in sodomy for pastime.
 slate.com
slate.com

Repeated anal sex (especially with the oversized penises women prefer) can harm the sphincter and rectum, leading to rectal prolapse and leakage/loose stools.
C. One reason women seek out and stay with attractive abusive men because they enjoy being beaten up.
Women seek out abusive (attractive) partners and stay with men who regularly beat them. In addition to psychological reasons, this is because the pain of getting physically assaulted turns them on. In fact, many women report having had the best sex of their lives immediately following such an “abusive episode.”
Studies on this phenomenon are suppressed and hidden by Google’s search algorithm.
D. Women engage in various paraphilias, including “knife play.”
Angelina Jolie reported that she could not reach orgasm during sex without being cut with a knife.
 www.dailymail.co.uk
www.dailymail.co.uk
Here, the actress is referring to a variation of “knife play,” a common fetish among women in which a sharp knife is inserted carefully into the vagina, causing targeted lacerations in the walls of the cervix and sending waves of sexual pleasure surging through the woman’s body.
***
In each of the above examples, harmful stimuli that should be perceived as dysphoric are misinterpreted by the female brain as euphoric; women feel pain as pleasure, even when their bodies are placed at harm’s risk.
From this, we can conclude there is a maladaptive mechanism in the female brain whereby pain is transformed into its polar opposite pleasure. Psychological aspects to this masochism exist as well, but the basis of this pleasure is neurological.
3. Counterpoint
Despite this, many studies deny the existence of this innate masochism in females, some even claiming that women suffer more pain than men.
Here is one such study:

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
In this study, an equal number of men and women were exposed to painful electrical stimuli of controlled strength.
Pupillary dilation was measured in both groups, and each participant was asked to rate on a decile scale how much pain they felt.
The female group presented with more pupillary dilation than the male group, and rated their pain higher than the men did. From this, the researchers concluded that women felt more pain than men.
But there are two obvious flaws with this methodology.
First, measuring pupillary dilation to quantify perceived pain is unreliable, since pupils dilate in response to both pain and pleasure. The dilation itself does not signify that specifically pain or pleasure is being felt, only that one of the two are. Naturally, the researchers assumed that the pupils dilated due to pain, but logically, pleasure cannot be ruled out.
Pupils also dilate in response to any strong emotion.
The second, and more detrimental, flaw was in asking the subjects to rate their pain themselves.
As researchers at UCLA (Toomey, et al) pointed out:
Asking subjects to rate their own pain runs into the same problem as the studies that compare the number of sex partners between men and women. In that case, men exaggerated their true number, while women minimized theirs. Here, we can be sure that the opposite will be the case. Women will pretend to feel great pain because of hypochondria and victim complex, whereas men will attempt to appear masculine by deliberately minimizing the amount of pain they feel.
Because of these flaws in methodology, the only valid conclusion we can derive from this study is that, when it comes to injury, women complain more than men.
***
A second argument is that women feel more pain than men due to having more nerves.

This argument also fails to convince.
Since my thesis is that pain is felt as pleasure to women, the more nerves a person has, the more able she is to feel not only pain, but also the concomitant pleasure. Increased nerve density increases potentiality of pleasure as well as pain. And if during a given pain event the pleasure overcrowds the pain, that intermingled feeling can’t be said to be pain at all, but pleasure, mathematically speaking. In a linear sense, pain you want to repeat over and over again due to the pleasure you get from it can’t be called pain. It's similar to the pleasure of repeatedly scratching a mosquito bite or bearing down on an aching tooth.
4. The Perception of Pain as Pleasure (In Women)
[...] that peak of sensitivity where the scarlet and the white threads of ultimate pain and ultimate joy are woven together...
- Yukio Mishima, Runaway Horses
I. Men experience pain in the analytical regions of the brain, whereas women experience “pain” in the emotional regions.
In 2008, researchers at UCLA conducted a study focusing on gender differences in pain perception:

 pdfs.semanticscholar.org
pdfs.semanticscholar.org
Here, researchers applied heat stimulations to the forearms of an equal number of male and female volunteers, while monitoring their brains using positron emission tomography (PET) scans.
The PET scans measured increases in blood flow and cerebral activation patterns during pain perception.
After analyzing the results, they concluded that “the cognitive, or analytic, region of the male brain lights up, while the female limbic system, the brain's emotional headquarters, springs into action.”

 science.howstuffworks.com
science.howstuffworks.com
Specifically, males had a larger magnitude of opioid receptor activation in the following areas:
1. Anterior thalamus
2. Hypothalamus
3. Ventral basal ganglia
While females had far more opioid receptor activation in the limbic system, comprised of:
1. Amygdala
2. Hypocampus
3. Thalamus
As in the previous study, the researchers noted that “the females verbally perceived the 50°C heat stimulus as more intense compared with males.”
The authors speculated that, because pain causes an emotionally charged limbic response in women, that may be responsible for the greater
complaining from the women. In other words, women felt “offended” by this pain, as if it were a social faux pas, and reacted similarly to it as if they had been hurt psychologically, rather than physically.
Even a foid scientist agrees:

According to Graham, nociceptive pain sensed in the limbic system (as it is in women) cannot be differentiated from psychological pain when examined through current medical technology.
Thus, increased activity in the limbic system in response to physical pain stimuli does not necessarily indicate a felt non-psychological pain.
This further suggests that, for women (but not men), physical pain is almost indistinguishable from social, psychological pain, such as being excluded, offended, outraged, or sad.
A woman, unable to distinguish between emotional and physical pain except through first causes, evidently wouldn’t be able to understand the distinction, no more than a blind man can comprehend the concept of vision.
II. Pain response is “mu-dominant” in females, while it is “kappa-dominant” in males.
When a nerve ending detects a painful stimulus, it sends a signal to the central nervous system, where the body produces calming and pain-relieving hormones called endorphins.
Endorphins (contracted from endogenous morphine) are a opioid-like substance similar to fentanyl that reduces pain in response to painful stimuli. Endorphins are “caught” by different opioid receptors in various parts of the brain, and produce varying effects depending on the type of opioid receptor they are caught by.
There are three different types of opioid receptors: mu, kappa, and delta.
Mu and kappas are the two most significant receptors; delta receptors have minimal effect in alleviating nociceptive pain.
Binding sites for the three receptors overlap in many brain structures, but some structures exhibit higher expression of one receptor over the others.
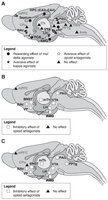
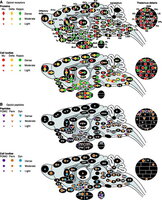
Here are some “brain maps” that show where mu- and kappa-opioid receptors are found in the brain.






As you can see, mu-opioid receptors are more prevalent in the limbic system, whereas kappa-opioid receptors predominate in the cognitive centers of the brain.
Specifically:
Mu is the most expressed opioid receptor in the amygdala, thalamus, mesencephalon and some brain stem nuclei. (1)
In a few structures, only one receptor type is detected: mu binding sites only are detected in four thalamic nuclei (lateral geniculate thalamus, ventrolateral thalamus, ventromedial thalamus, and posterior thalamus), the sensory trigeminal nucleus (SNT) and nucleus ambiguus (Amb).
Kappa is the most represented receptor in the basal anterior forebrain, including the claustrum (Cl) and endopiriform cortex (En), olfactory tubercle (Tu), striatum (caudate putamen and nucleus accumbens), preoptic area (POA), hypothalamus, and pituitary.
Kappa binding sites only are found in seven brain regions that are part of the stress axis (Cl, paraventricular hypothalamus, arcuate nucleus, supraoptic nucleus, Me, CeA, and pituitary).
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482114/
A Yale study similarly found mu-opioid dominance in females and kappa-receptor dominance in males.
Mu-opioid dominance is also confirmed by better reaction from females to mu-opioid-specific drugs than men. Women required 40% less morphine (a mu-opioid-specific medicine) than men for post-operative, reardless of body weight or diffusion of the drug in the body.
Women also seem to get much greater pain relief from mixed-action opioid medications and experience greater pain relief with mu-specific opioids.
Based on these findings, researchers concluded that that mu-opioid receptor (MOR) binding density would be higher in females than in males.
III. Mu are the rewarding receptors, while kappa are the punishing receptors.
Mu and kappa receptors both serve a role in mediating nociceptive pain, but do so in different ways. To put it simply, mu-opioid receptors “reward” pain, whereas kappa receptors “punish” it.
The functional response of mu- and kappa-opioid receptors can be replicated through the administration of mu-specific or kappa-specific drugs. The pain response of opioid receptors can be emulated to a greater or lesser extent by administering these drugs and observing their effects.
In other words, the effects caused by theses receptor-specific agonists are the same (differering only in degree) as the “natural” response to pain caused by non-artificial means of activation (i.e. actual pain stimuli, rather than induced activation).
A. Mu Receptors
The areas in which mu-opioid receptors are most prevalent (the limbic system, the primary regions in which females register pain) are known informally as “hedonistic hotspots.” These areas of the brain play a large role in the reinforcement of pleasure. Overactivation of this area are drug addiction, food addiction, etc.
These receptors contribute to the reinforcing properties of most drugs of abuse.
Stimulation of mu opioid receptors generates an increase in both “liking” and “wanting” for reward.
Thus, mu-opioid receptors induce relaxation, trust, satisfaction and have a strong analgesic effect.
Morphine is a powerful pain relieving drug that produces euphoria. It is mu-opioid specific, meaning it acts on and targets the mu-opioid receptors. Other mu-specific drugs include heroin and fentanyl. Most opioid drugs of abuse fall under this category.
The effects of morphine include euphoria, mood lift, relaxation, and analgesia.
B. Kappa Receptors
Kappa is generally known to be a dysphoric, aversive receptor in terms of its pain-mediating effects.
While systemic mu agonists (morphine, heroin, etc) produce positive reinforcement, kappa agonists induce aversion, hallucinations, and malaise, producing anxiety, fear, and depression.
Furthermore, activation of kappa receptors counteract the reward processes of the mu-receptors, and in male models, increases psychological discomfort associated with pain.
Because of these dysphoric effects, no kappa-specific drug is in wide use today, either as anaesthesia or recreationally. Mixed-action opioids, however, such as nalbuphine, have been used on women to some success, although they are ineffective in men (See Section IV).
Kappa receptors are the “punishing” receptors.
It is believed that kappa-opioid receptors exist to produce avoidance behaviors in response to pain, causing extreme negative feelings to be associated with the source of the pain, so that the brain can learn to avoid similar sources of pain in the future.
***
In short, MOR (mu-opioid receptors) produce a euphoric effect in response to pain, similar to that of fentanyl, heroin, or morphine.
KOR (kappa-opioid receptors), however, produce a dysphoric effect, causes psychological discomfort, anxiety, fear, and depression.
IV. Kappa receptors work synergistically with mu receptors in females, increasing the “rewarding” effect. In contrast, the two receptors work at cross purposes in males; kappa decreases the effect of mu receptors in men, thereby increasing the “punishing” effect.
In Section III, it has been established that kappa receptors are punishing and dysphoric. New studies have suggested, however, that the negative effects of KOR activation seems to only be present in males. In females, kappa receptor activation appears to have a synergistic effect with mu-opioid receptors, making the “rewarding” aspects of it more powerful.
Because kappa-opioids had mostly been tested on male subjects, on whom kappa activation results in dysophoria and great mental distress, medical professionals traditionally have dismissed kappa-opioids as viable analgesics in humans.
However, UCSF scientists recently performed a study about the analgesic effects of kappa agonists, this time on human female subjects.
They undertook this after a previous study discovered that kappa-opioids brought pain relief to female rats but not male rats.
They found that in women, a drug made up of a strong concentration of kappa-opioid has a strong and lasting analgesic effect.
In contrast, in men, the low dose actually increased pain; as the dose was increased, the heightened pain disappeared and a weak, short-lived analgesic effect set in.
In their previous study on rats, the Fields team showed that treating the vlPAG neurons of male rats with a mu opioid brought about pain relief, but that subsequently adding kappa-opioid into the RVM markedly decreased the mu opioid’s analgesic effect.
In males, KOR worked to sabotage MOR in a sense, and decreased the rewards of mu.
However, Treating the vlPAG neurons of female rats with a mu opioid brought on the expected pain relief, but subsequently adding kappa-opioid into the RVM increased the mu opioid’s analgesic effect.
What these studies show is that mu- and kappa-opioid receptors work at cross-purposes in males, decreasing the rewarding effects of mu receptors.
However, the two receptors work synergistically in females; kappa activation increases the rewarding effects of mu receptors.
V. Estrogen is a natural painkiller that modulates opioid receptors to be more effective.
Estrogen is present in women at a level that is 10 to 15 times higher than the level present in men.
In 2011, researchers (Stenning et al) discovered that estrogen can modulate the density of opioid receptors.
Estrogen have also been found to induce mu-opioid receptor activation within the preoptic nucleus and posterodorsal medial amygdaloid nucleus.
High densities of estrogen receptors functionally related to endorphin receptors have been found within the hypothalamus, one of the primary sites of MOR. [mu-opioid receptors]
In addition, research on sex hormones indicates there is improved mu receptor binding in some brain regions in women after they receive estrogen, as measured by PET scans.
One of the conclusions forwarded from this research is that a decrease in estrogen would increase sensitivity to pain. Conversely, an increase in estrogen would promote analgesic effect by stimulating a bolus of pain-inhibiting transmitters.
The effects of estrogen modulation of pain receptors is further confirmed in a study by Dr. Kern Olson.
According to him:
Girls and boys react to pain in a similar fashion before puberty but differently after puberty; these differences, however, decrease as levels of sex hormones decrease as people age. (i.e., after menopause, when estrogen levels in women plummet)

 www.practicalpainmanagement.com
www.practicalpainmanagement.com
VI. Opioid receptor activation is anti-sexual in men, pro-sexual in women.
According to the same study above:
On a study on rats, an injection of mu-specific opioid drugs into the brains of male rats were seen to suppress male gonadal function. In other words, the rats were unable to maintain an erection after being administered these drugs, and mating behaviors decreased in response.
However, in female rats, administration of mu-specific drugs did not correlate with symptoms of sexual dysfunction.
On the contrary, when mu-opioids were administrated to female rats only in a group, unpaced mating increased. This nymphomania was increased the closer the rats got to estrus, corresponding with higher levels of estrogen. This was associated to higher rates of mu activation, which suggests a certain threshold of pain must be reached before sexual arousal happens.
This suggests that the pro-sexual effect of MOR activation on females will increase when estrogen levels are higher, such as during estrus, certain phases of the menstrual cycle, and the later terms of pregnancy.
The researchers reported that with lower levels of estrogen, progesterone was pronociceptive.
Thresholds were decreased, and pain intensities increased during the midluteal phase when progesterone levels were relatively higher than estrogen levels.
This pro-sexual phenomenon can be observed in human females as well. Estrogen serves to increase the feelings of sexual arousal derived from pain.
This effect can be seen in the “birthgasm.” During pregnancy, the estrogen levels in a women increase the closer she gets to giving birth. This effect is seen during pregnancy and may account for pregnancy-induced increases in tolerance to nociception. This may have evolved so that a woman could better tolerate the “pain” of childbirth. As a result, a woman’s estrogen levels are necessarily highest just before and during the act of giving birth. Estrogen and MOR activation working in tandem have often resulted in women having orgasms during childbirth:
When a woman feels the contractions of an orgasm and/or extreme moments of pleasure right at the moment of delivering her baby, this may be called an “orgasmic birth.” You may feel tremendous pressure and sensation in the vagina as your baby's birth approaches, then a powerful, pleasurable release that's both physical and orgasmic.
This phenomenon is common enough that the term of “birthgasm” was coined.
5. Acupuncture
Acupuncture was a saving grace. It helps more than anything else I tried.
- Molly Qerim, a foid
***
Acupuncture is a form of alternate medicine in which thin needles are inserted into the body. The practice of acupuncture is considered a pseudoscience because the theories and practices of traditional Chinese medicine -- based on the concepts of qu, meridians, and acupuncture points, life force energy -- are not amenable to modern scientific knowledge, and it has been characterized as quackery.
Many scientific reviews have found that acupuncture is ineffective for a wide range of conditions.
Some research results suggest that it can alleviate some forms of pain, though the majority of research suggests that its apparent effects are not caused by the treatment itself. Many acupunctures attribute pain relief to the release of endorphins when needles penetrate.

 en.wikipedia.org
en.wikipedia.org
In other words, there is a prevailing theory that the way acupuncture “works” is by causing pain that causes the body to go into pain control overdrive, releasing endorphins and creating a state of well-being.
Unsurprisingly, the great majority of proponents of acupuncture are female. By some estimates, the ratio of women to men who use acupuncture regularly is 5 to 1. (This especially applies to East Asian women, who are on average more masochistic and exogamic than women of other races.) Discounting the placebo effect, the logical explanation is that acupuncture works for women, but not men.

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
To see why this is, let’s examine the differences of male and female brains’ reactions in response to acupuncture.
In males:
The needle is inserted.
Pain is sensed by the nerves.
A small number of rewarding mu receptors activate, and a large number of punishing kappa receptors activate.
Mu receptors try to “reward” for the pain. However, the kappa receptors lessen this effect.
Male feels pain and aversion. He begins to associate acupuncture with negative feelings of dysphoria.
The man’s sexual desire decreases, and erection becomes impossible.
In females:
The needle is inserted.
Pain is sensed by the nerves.
A large number of rewarding mu receptors activate, and a small number of synergistically rewarding kappa receptors activate.
Mu rewards the woman for the pain. Kappa helps this effect, transforming pain into pleasure.
Estrogen modulates the pain response, causing sexual arousal.
Female feels euphoria and sexual arousal. She begins to associate acupuncture with positive feelings of euphoria.
The woman’s sexual desire increases; her desire for unpaced mating goes up.
It’s not difficult to see, then, why women are the primary consumers of acupuncture.
***
A famous example of acupuncture working as advertised proves my point further.
In the early 20th century, surgeons performed open heart surgery on a 15-year-old girl in China without using anesthesia. The only measures taken to alleviate the pain of the surgery was acupuncture. Nevertheless, it is said that girl remained calm and immobile during the operation, which was a great success. To this day, this is held up as one of the primary pieces evidence of evidence in support of the efficacy of acupuncture.
However, notice that it was a girl who received the surgery, and not a boy. A male would scarcely have been able to endure the pain of the operation. However, it would have been no difficulty at all for a female.

6. Conclusion
Proposed Changes to Society:
1. Medication.
Opioids and anesthetics are inherently dangerous to administer to women, since there is a greater chance of overdose and addiction. (See III.A)
As a result, it would be beneficial for their safety if anesthetics were not administered during female patients’ surgeries.
Anesthesia can be replaced with acupuncture for female patients, which, as shown above (5), has comparable efficacy and far less risk.
This will also increase supply and decrease demand of anesthesia medication, leading to a decrease in the cost of expensive anesthesia procedures.

 www.practicalpainmanagement.com
www.practicalpainmanagement.com
2. Abortion.
Many current proponents of abortion justify the legality of early-term abortion by the logic that the baby does not yet feel pain.
Following this logic, it would seem that crimes are more serious the more pain is caused.
But women essentially feel no physical pain, as shown above.
To maintain legal consistency, it follows that either abortion must be classed as murder, or crimes that cause bodily injury (battery and domestic assault) to women should not be punished as harshly as those which cause bodily injury to men.
3. Scientific Studies.
German scientists and physicians made great medical advances in the 1940s, due to their progressive genetic research and innovative surgical experimentation, particularly in the area of twins. This was a time of medical enlightenment, and many of the concepts they discovered are still used today. Various legislative considerations, however, prevent us from conducting similar studies today.
However, since our knowledge of the nature of human suffering was then incomplete and now we are more informed, some of these restrictions can now be loosened on experimentation on female subjects.
@ShadowTheEdgehog
@Mainländer
1. Introduction
Joan of Arc at the Coronation of Charles VII (1854), Jean Auguste Dominque Ingres.
Joan of Arc (1412-31) was an historical foid who lived during the Hundred Years’ War, a great conflict between France and England.
Born in the peaceful French village of Domrémy, Joan suffered from schizophrenic delusions since early childhood. At the age 17, she hallucinated that an evil spirit was instructing her to lead France’s army to victory over the English. She was so insistent about this that she was granted an audience with the royal court. Upon hearing her prophecy, the imbecilic King of France, Dauphin Charles VII, took her at her word and placed her in command of a battalion of soldiers.
Joan’s first military campaigns were successful. In 1429, Joan and her soldiers relieved the besieged French at the Siege of Orleans, earning her the title of “The Maid of Orleans.” In May, 1430, however, her fortunes changed dramatically and she was routed and captured by the English in Compiègne. After a short trial, she was sentenced to death by burning at for the crime of witchcraft.
Thousands of people witnessed Joan’s execution, and many accounts of her death survive to this day. As she burned at the stake, the following signs were observed:
1. Her muscles contracted and tensed up.
2. Her breasts became full and began to heave violently.
3. She cast her eyes up to heaven, and her expression was not one of pain, but something approaching spiritual ecstasy.
4, In the instant before she lost consciousness, Joan moaned out Jesus’s name three times, loud enough to be heard over the roar of the flames.
5. Joan’s vagina, in contrast to the dry kindling that burned around her, became lubricated and wet.
Individually, each of these signs do not point to a clear conclusion. But taken together, they indicate an undeniable certainty: as the flames enfolded and consumed her body, Joan of Arc was caught within the grips of a powerful orgasm.
2. Masochism
Masochism is defined as “the condition of experiencing recurring and intense sexual arousal in response to enduring moderate or extreme pain, suffering, or humiliation.” It’s described as an addiction-like tendency, with features resembling drug addiction: craving, intoxication, tolerance, and withdrawal.
Masochism is seen as aberrant in males and treated as a harmful psychological disorder.
Female masochism, on the other hand, does not suffer from the same stigma, given its ubiquity.
“Masochism is more prevalent among women than men. [...] Such things as beating fantasies and self-punishment are phenomena that seem to be more common among women than among men.''
- Dr. Eleanor Galenson, Professor of Psychiatry at the Mount Sinai School of Medicine
Confirming this, In Three Essays on the Theory of Sexuality (1924), Sigmund Freud described the three essential traits of femininity -- narcissism, passivity, and masochism. To Freud, pain was an inseparable part of the intensity of a woman’s sexual pleasure, “an expression of the feminine being nature.” He found in women a persistent need for punishment and humiliation, "which succeed [...] in binding erotically the destructive trends which have been diverted inwards."
There are numerous examples of masochism in females:
A. Women overwhelmingly prefer larger-than-average penises.
Here are some quotes from a VICE article:
Alicia is immensely turned on by dick-related pain during the act itself. “I enjoy pain during sex, so I do really like that initial pain of a huge cock smashing my cervix,” Alicia explains. The first time she felt a dick hit her cervix, she was super turned on and already past the point where pain feels painful. “I normally orgasm fairly easily, but this was something else,” she recounts. “My legs were shaking, and after we were done my legs were weak for quite a while. So, intense orgasms were achieved, with seemingly much less effort on his part compared to what I've experienced with smaller partners.”
This feeling is shared by Hanna, who thrives on the challenge of being able to take a massive member. “It gives me a strange sense of pride. I love the feeling of being totally stretched out and the reminder the next day if I'm sore.”

We Spoke to Size Queens About Why They Prefer Big Dicks
“It gives me a strange sense of pride. I love the feeling of being totally stretched out.”
The top selling dildos on the internet are eight to nine inches long. In the absence of a large cock, some women resort to bestiality with dogs and horses, animals with large cocks.
However, a penis of this size inflicts injuries and perforations on the vagina and causes lasting damage in the form of tears in the cervix, as well as dilation of the vaginal opening. Infections can occur, and there are several case reports of pelvic abscess and subsequent scarring due to insertion of large objects into the vagina.
B. Women love anal sex, despite the lack of an erogenous zone there.
Homosexual men also engage in anal sex. But keep in mind that gay men have erogenous zones deep within their ani known as prostate glands. Women lack this, and so in theory anal sex should be not pleasurable for them, and instead extremely painful. Nevertheless, women engage in sodomy for pastime.
94 percent of women who received anal sex in their last encounter said they reached orgasm-a higher rate of orgasm than was reported by women who had vaginal intercourse or received oral sex.

Why Do Women Who Have Anal Sex Get More Orgasms?
94 percent of women who received anal sex in their last encounter said they reached orgasm.

Repeated anal sex (especially with the oversized penises women prefer) can harm the sphincter and rectum, leading to rectal prolapse and leakage/loose stools.
C. One reason women seek out and stay with attractive abusive men because they enjoy being beaten up.
Women seek out abusive (attractive) partners and stay with men who regularly beat them. In addition to psychological reasons, this is because the pain of getting physically assaulted turns them on. In fact, many women report having had the best sex of their lives immediately following such an “abusive episode.”
Studies on this phenomenon are suppressed and hidden by Google’s search algorithm.
D. Women engage in various paraphilias, including “knife play.”
Angelina Jolie reported that she could not reach orgasm during sex without being cut with a knife.
Angelina Jolie reveals her dark sexual exploits
Mother of four Angelina Jolie has spoken openly about her dark sexual past. The Hollywood heavyweight says she used a knife to cut herself and her early boyfriend, after losing her virginity at 14
Here, the actress is referring to a variation of “knife play,” a common fetish among women in which a sharp knife is inserted carefully into the vagina, causing targeted lacerations in the walls of the cervix and sending waves of sexual pleasure surging through the woman’s body.
***
In each of the above examples, harmful stimuli that should be perceived as dysphoric are misinterpreted by the female brain as euphoric; women feel pain as pleasure, even when their bodies are placed at harm’s risk.
From this, we can conclude there is a maladaptive mechanism in the female brain whereby pain is transformed into its polar opposite pleasure. Psychological aspects to this masochism exist as well, but the basis of this pleasure is neurological.
3. Counterpoint
Despite this, many studies deny the existence of this innate masochism in females, some even claiming that women suffer more pain than men.
Here is one such study:

Gender differences in pain ratings and pupil reactions to painful pressure stimuli - PubMed
In order to investigate gender differences in pain perception, the present study employed both a psychophysical and a psychophysiological measure. In experiment 1, 20 subjects rated the painfulness of 4 different levels of tonic pressure applied to their fingers using a verbally anchored...
In this study, an equal number of men and women were exposed to painful electrical stimuli of controlled strength.
Pupillary dilation was measured in both groups, and each participant was asked to rate on a decile scale how much pain they felt.
The female group presented with more pupillary dilation than the male group, and rated their pain higher than the men did. From this, the researchers concluded that women felt more pain than men.
But there are two obvious flaws with this methodology.
First, measuring pupillary dilation to quantify perceived pain is unreliable, since pupils dilate in response to both pain and pleasure. The dilation itself does not signify that specifically pain or pleasure is being felt, only that one of the two are. Naturally, the researchers assumed that the pupils dilated due to pain, but logically, pleasure cannot be ruled out.
Pupils also dilate in response to any strong emotion.
The second, and more detrimental, flaw was in asking the subjects to rate their pain themselves.
As researchers at UCLA (Toomey, et al) pointed out:
Women may report more pain than men due to social conditioning and gender expectations. Gender roles permit girls to be emotional and express pain openly, whereas boys are expected to be brave and stoic and keep a stiff upper lip.
Asking subjects to rate their own pain runs into the same problem as the studies that compare the number of sex partners between men and women. In that case, men exaggerated their true number, while women minimized theirs. Here, we can be sure that the opposite will be the case. Women will pretend to feel great pain because of hypochondria and victim complex, whereas men will attempt to appear masculine by deliberately minimizing the amount of pain they feel.
Because of these flaws in methodology, the only valid conclusion we can derive from this study is that, when it comes to injury, women complain more than men.
***
A second argument is that women feel more pain than men due to having more nerves.

This argument also fails to convince.
Since my thesis is that pain is felt as pleasure to women, the more nerves a person has, the more able she is to feel not only pain, but also the concomitant pleasure. Increased nerve density increases potentiality of pleasure as well as pain. And if during a given pain event the pleasure overcrowds the pain, that intermingled feeling can’t be said to be pain at all, but pleasure, mathematically speaking. In a linear sense, pain you want to repeat over and over again due to the pleasure you get from it can’t be called pain. It's similar to the pleasure of repeatedly scratching a mosquito bite or bearing down on an aching tooth.
4. The Perception of Pain as Pleasure (In Women)
[...] that peak of sensitivity where the scarlet and the white threads of ultimate pain and ultimate joy are woven together...
- Yukio Mishima, Runaway Horses
I. Men experience pain in the analytical regions of the brain, whereas women experience “pain” in the emotional regions.
In 2008, researchers at UCLA conducted a study focusing on gender differences in pain perception:

Course Update for Nurse Anesthetists Gender Differences in Pain : Does X = Y ? | Semantic Scholar
The aims of this course are to update anesthesia providers about the differences between genders in pain sensitivity and treatment and to elucidate the complex aspects of the biology of such differences. AANA Journal October 2008 Vol. 76, No. 5 355 Increasing evidence suggests that men and women...
Here, researchers applied heat stimulations to the forearms of an equal number of male and female volunteers, while monitoring their brains using positron emission tomography (PET) scans.
The PET scans measured increases in blood flow and cerebral activation patterns during pain perception.
After analyzing the results, they concluded that “the cognitive, or analytic, region of the male brain lights up, while the female limbic system, the brain's emotional headquarters, springs into action.”

Do men and women feel pain differently?
Studies show that women are more sensitive to pain than men, despite their bodies' ability to withstand the agony of childbirth. Does social conditioning help men keep a stiff upper lip when they're hurt? Or do emotions and estrogen factor into this painful equation?
Specifically, males had a larger magnitude of opioid receptor activation in the following areas:
1. Anterior thalamus
2. Hypothalamus
3. Ventral basal ganglia
While females had far more opioid receptor activation in the limbic system, comprised of:
1. Amygdala
2. Hypocampus
3. Thalamus
As in the previous study, the researchers noted that “the females verbally perceived the 50°C heat stimulus as more intense compared with males.”
The authors speculated that, because pain causes an emotionally charged limbic response in women, that may be responsible for the greater
complaining from the women. In other words, women felt “offended” by this pain, as if it were a social faux pas, and reacted similarly to it as if they had been hurt psychologically, rather than physically.
The authors assimilated this difference with greater activation in the thalamus, anterior insula, and contralateral prefrontal cortex of females as evidenced by the PET scan. The difference found within the prefrontal cortex may be responsible for the affective, or psychological, differences seen between genders in pain perception.
Even a foid scientist agrees:
"Pain is inherently subjective," says Jennifer Graham. "We typically rely on self-report to know if someone is experiencing it." And it's tough to determine how much of pain is sensory and how much is influenced by psychological factors, she adds. "The limbic system of the brain, which is related to emotion, is typically active in response to physical pain for both men and women. In fact, looking at functional MRI, it can be difficult to distinguish psychological pain-such as that caused by social exclusion-from pain that is purely physical."
Gender Differences In Brain Response To Pain
A new UCLA study shows that different parts of the brain are stimulated in reaction to pain depending on gender. The research, which represents the largest gender-comparison study of its kind, focused on people with irritable bowel syndrome (IBS), one of the nation's most common chronic medical...
www.sciencedaily.com
According to Graham, nociceptive pain sensed in the limbic system (as it is in women) cannot be differentiated from psychological pain when examined through current medical technology.
Thus, increased activity in the limbic system in response to physical pain stimuli does not necessarily indicate a felt non-psychological pain.
This further suggests that, for women (but not men), physical pain is almost indistinguishable from social, psychological pain, such as being excluded, offended, outraged, or sad.
A woman, unable to distinguish between emotional and physical pain except through first causes, evidently wouldn’t be able to understand the distinction, no more than a blind man can comprehend the concept of vision.
II. Pain response is “mu-dominant” in females, while it is “kappa-dominant” in males.
When a nerve ending detects a painful stimulus, it sends a signal to the central nervous system, where the body produces calming and pain-relieving hormones called endorphins.
Endorphins (contracted from endogenous morphine) are a opioid-like substance similar to fentanyl that reduces pain in response to painful stimuli. Endorphins are “caught” by different opioid receptors in various parts of the brain, and produce varying effects depending on the type of opioid receptor they are caught by.
There are three different types of opioid receptors: mu, kappa, and delta.
Mu and kappas are the two most significant receptors; delta receptors have minimal effect in alleviating nociceptive pain.
Binding sites for the three receptors overlap in many brain structures, but some structures exhibit higher expression of one receptor over the others.
Here are some “brain maps” that show where mu- and kappa-opioid receptors are found in the brain.
As you can see, mu-opioid receptors are more prevalent in the limbic system, whereas kappa-opioid receptors predominate in the cognitive centers of the brain.
Specifically:
Mu is the most expressed opioid receptor in the amygdala, thalamus, mesencephalon and some brain stem nuclei. (1)
In a few structures, only one receptor type is detected: mu binding sites only are detected in four thalamic nuclei (lateral geniculate thalamus, ventrolateral thalamus, ventromedial thalamus, and posterior thalamus), the sensory trigeminal nucleus (SNT) and nucleus ambiguus (Amb).
Kappa is the most represented receptor in the basal anterior forebrain, including the claustrum (Cl) and endopiriform cortex (En), olfactory tubercle (Tu), striatum (caudate putamen and nucleus accumbens), preoptic area (POA), hypothalamus, and pituitary.
Kappa binding sites only are found in seven brain regions that are part of the stress axis (Cl, paraventricular hypothalamus, arcuate nucleus, supraoptic nucleus, Me, CeA, and pituitary).
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482114/
A Yale study similarly found mu-opioid dominance in females and kappa-receptor dominance in males.
Males had significantly higher V T and thus a higher KOR (kappa opioid receptor) availability than women in multiple brain regions.
Mu-opioid dominance is also confirmed by better reaction from females to mu-opioid-specific drugs than men. Women required 40% less morphine (a mu-opioid-specific medicine) than men for post-operative, reardless of body weight or diffusion of the drug in the body.
Women also seem to get much greater pain relief from mixed-action opioid medications and experience greater pain relief with mu-specific opioids.
Based on these findings, researchers concluded that that mu-opioid receptor (MOR) binding density would be higher in females than in males.
In addition, the idea that males and females respond differently to opioids is not new, but until recently the difference was believed to be limited to potency, with clinical studies showing that women require less morphine for post-operative pain than men.
(4) Research by Craft found that women use 40% less opioid-based medicine than men for postoperative pain.10 This finding was confirmed by Miaskowski et al in an analysis of 18 studies of postoperative opioid use.
Several studies of pain after oral surgery revealed that women get much greater pain relief from mixed-action opioid medications (eg, pentazocine, nalbuphine, butorphanol).12 More recently, a meta-analysis of this literature confirmed that women seem to experience greater pain relief with opioids.
In their study, the researchers also discovered that female rats received significantly more pain relief when mu opioid was injected into the vlPAG than male rats, a fact that was not attributable to body weight or diffusion of the drug in the body.
***
Moreover, men are more likely than women to engage in substance abuse (Lynch et al. 2002), while women become addicted to opiates more quickly following first use (Lex 1991; Roth et al. 2004). Similarly, female rats acquired heroin self-administration more quickly than their male counterparts, and subsequently, self-administered larger amounts of the drug (Lynch and Carroll 1999; Cicero et al. 2003). It is plausible that sex differences in MOR activation underlie sex differences in these behaviors. In support, PET scan studies revealed higher MOR binding in several brain regions of women compared to men (Zubieta et al. 1999). Likewise, higher MOR binding density was found in several brain regions in female rats compared to males, although these rats were gonadectomized (Vathy et al. 2003). However, it remains unknown whether sex differences are present in the intact rat brain and whether these sex differences emerge early in development. Therefore, our second aim was to compare MOR binding density between intact male and female rats at both juvenile and adult ages. Based on these previous findings in humans and gonadectomized adult rats (Zubieta et al. 1999; Vathy et al. 2003), we hypothesized that MOR binding density would be higher in females than in males.
(4) Research by Craft found that women use 40% less opioid-based medicine than men for postoperative pain.10 This finding was confirmed by Miaskowski et al in an analysis of 18 studies of postoperative opioid use.
Several studies of pain after oral surgery revealed that women get much greater pain relief from mixed-action opioid medications (eg, pentazocine, nalbuphine, butorphanol).12 More recently, a meta-analysis of this literature confirmed that women seem to experience greater pain relief with opioids.
In their study, the researchers also discovered that female rats received significantly more pain relief when mu opioid was injected into the vlPAG than male rats, a fact that was not attributable to body weight or diffusion of the drug in the body.
***
Moreover, men are more likely than women to engage in substance abuse (Lynch et al. 2002), while women become addicted to opiates more quickly following first use (Lex 1991; Roth et al. 2004). Similarly, female rats acquired heroin self-administration more quickly than their male counterparts, and subsequently, self-administered larger amounts of the drug (Lynch and Carroll 1999; Cicero et al. 2003). It is plausible that sex differences in MOR activation underlie sex differences in these behaviors. In support, PET scan studies revealed higher MOR binding in several brain regions of women compared to men (Zubieta et al. 1999). Likewise, higher MOR binding density was found in several brain regions in female rats compared to males, although these rats were gonadectomized (Vathy et al. 2003). However, it remains unknown whether sex differences are present in the intact rat brain and whether these sex differences emerge early in development. Therefore, our second aim was to compare MOR binding density between intact male and female rats at both juvenile and adult ages. Based on these previous findings in humans and gonadectomized adult rats (Zubieta et al. 1999; Vathy et al. 2003), we hypothesized that MOR binding density would be higher in females than in males.
III. Mu are the rewarding receptors, while kappa are the punishing receptors.
Mu and kappa receptors both serve a role in mediating nociceptive pain, but do so in different ways. To put it simply, mu-opioid receptors “reward” pain, whereas kappa receptors “punish” it.
The functional response of mu- and kappa-opioid receptors can be replicated through the administration of mu-specific or kappa-specific drugs. The pain response of opioid receptors can be emulated to a greater or lesser extent by administering these drugs and observing their effects.
In other words, the effects caused by theses receptor-specific agonists are the same (differering only in degree) as the “natural” response to pain caused by non-artificial means of activation (i.e. actual pain stimuli, rather than induced activation).
A. Mu Receptors
The areas in which mu-opioid receptors are most prevalent (the limbic system, the primary regions in which females register pain) are known informally as “hedonistic hotspots.” These areas of the brain play a large role in the reinforcement of pleasure. Overactivation of this area are drug addiction, food addiction, etc.
These receptors contribute to the reinforcing properties of most drugs of abuse.
Stimulation of mu opioid receptors generates an increase in both “liking” and “wanting” for reward.
Thus, mu-opioid receptors induce relaxation, trust, satisfaction and have a strong analgesic effect.
Morphine is a powerful pain relieving drug that produces euphoria. It is mu-opioid specific, meaning it acts on and targets the mu-opioid receptors. Other mu-specific drugs include heroin and fentanyl. Most opioid drugs of abuse fall under this category.
The effects of morphine include euphoria, mood lift, relaxation, and analgesia.
Relevant to drug intake, genetic data demonstrate that mu receptors contribute to the reinforcing properties of most drugs of abuse, [...]
The opioid system, which mediates hedonic evaluation of natural rewards, represents another key substrate for the deleterious effects of drugs of abuse. Indeed, the reinforcing properties of many abused drugs depend on the activation of mu opioid receptors
which thus may be a potential molecular gateway to drug addiction (72).
Conversely, mu and delta antagonists [medications which inhibit the effect of these receptors] suppress the positive reinforcing properties of natural rewards and opiate or nonopioid drugs, whereas kappa receptors induce dysphoria and counteract mu receptors in regulating hedonic homeostasis. (2)
SOURCE: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482114/
The opioid system, which mediates hedonic evaluation of natural rewards, represents another key substrate for the deleterious effects of drugs of abuse. Indeed, the reinforcing properties of many abused drugs depend on the activation of mu opioid receptors
which thus may be a potential molecular gateway to drug addiction (72).
Conversely, mu and delta antagonists [medications which inhibit the effect of these receptors] suppress the positive reinforcing properties of natural rewards and opiate or nonopioid drugs, whereas kappa receptors induce dysphoria and counteract mu receptors in regulating hedonic homeostasis. (2)
SOURCE: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482114/
B. Kappa Receptors
Kappa is generally known to be a dysphoric, aversive receptor in terms of its pain-mediating effects.
While systemic mu agonists (morphine, heroin, etc) produce positive reinforcement, kappa agonists induce aversion, hallucinations, and malaise, producing anxiety, fear, and depression.
Furthermore, activation of kappa receptors counteract the reward processes of the mu-receptors, and in male models, increases psychological discomfort associated with pain.
Because of these dysphoric effects, no kappa-specific drug is in wide use today, either as anaesthesia or recreationally. Mixed-action opioids, however, such as nalbuphine, have been used on women to some success, although they are ineffective in men (See Section IV).
Kappa receptors are the “punishing” receptors.
It is believed that kappa-opioid receptors exist to produce avoidance behaviors in response to pain, causing extreme negative feelings to be associated with the source of the pain, so that the brain can learn to avoid similar sources of pain in the future.
Pharmacological studies have long shown that kappa receptor activation is aversive in animal models.
Globally systemic mu agonists produce positive reinforcement, whereas kappa agonists induce aversion, hallucinations, and malaise.
Kappa receptors also counteract reward processes under stressful conditions.
Traditionally, kappa-opioids have been dismissed as ineffective analgesics in humans.
Nalbuphine, which is often used to anesthetize women during childbirth, does little to mitigate pain in men.
Kappa receptors induce dysphoria and counteract mu receptors in regulating hedonic homeostasis. (2)
SOURCE: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482114/
KOR agonists produce signs of anxiety, fear, and depression in laboratory animals and humans, findings that have led to the hypothesis that drug withdrawal-induced DYN release is instrumental in negative reinforcement processes that drive addiction. However, these studies were almost exclusively conducted in males.

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
Globally systemic mu agonists produce positive reinforcement, whereas kappa agonists induce aversion, hallucinations, and malaise.
Kappa receptors also counteract reward processes under stressful conditions.
Traditionally, kappa-opioids have been dismissed as ineffective analgesics in humans.
Nalbuphine, which is often used to anesthetize women during childbirth, does little to mitigate pain in men.
Kappa receptors induce dysphoria and counteract mu receptors in regulating hedonic homeostasis. (2)
SOURCE: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482114/
KOR agonists produce signs of anxiety, fear, and depression in laboratory animals and humans, findings that have led to the hypothesis that drug withdrawal-induced DYN release is instrumental in negative reinforcement processes that drive addiction. However, these studies were almost exclusively conducted in males.

Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction
Behavioral, biological, and social sequelae that lead to drug addiction differ between men and women. Our efforts to understand addiction on a mechanistic level must include studies in both males and females. Stress, anxiety, and depression are tightly ...
***
In short, MOR (mu-opioid receptors) produce a euphoric effect in response to pain, similar to that of fentanyl, heroin, or morphine.
KOR (kappa-opioid receptors), however, produce a dysphoric effect, causes psychological discomfort, anxiety, fear, and depression.
IV. Kappa receptors work synergistically with mu receptors in females, increasing the “rewarding” effect. In contrast, the two receptors work at cross purposes in males; kappa decreases the effect of mu receptors in men, thereby increasing the “punishing” effect.
In Section III, it has been established that kappa receptors are punishing and dysphoric. New studies have suggested, however, that the negative effects of KOR activation seems to only be present in males. In females, kappa receptor activation appears to have a synergistic effect with mu-opioid receptors, making the “rewarding” aspects of it more powerful.
Because kappa-opioids had mostly been tested on male subjects, on whom kappa activation results in dysophoria and great mental distress, medical professionals traditionally have dismissed kappa-opioids as viable analgesics in humans.
However, UCSF scientists recently performed a study about the analgesic effects of kappa agonists, this time on human female subjects.
They undertook this after a previous study discovered that kappa-opioids brought pain relief to female rats but not male rats.
They found that in women, a drug made up of a strong concentration of kappa-opioid has a strong and lasting analgesic effect.
In contrast, in men, the low dose actually increased pain; as the dose was increased, the heightened pain disappeared and a weak, short-lived analgesic effect set in.
In their previous study on rats, the Fields team showed that treating the vlPAG neurons of male rats with a mu opioid brought about pain relief, but that subsequently adding kappa-opioid into the RVM markedly decreased the mu opioid’s analgesic effect.
In males, KOR worked to sabotage MOR in a sense, and decreased the rewards of mu.
However, Treating the vlPAG neurons of female rats with a mu opioid brought on the expected pain relief, but subsequently adding kappa-opioid into the RVM increased the mu opioid’s analgesic effect.
Traditionally, kappa-opioids have been dismissed as ineffective analgesics in humans.
While the majority of sex difference findings related to KOR are from studies of the analgesic effects of kappa agonists, there is also emerging evidence of KOR-related sex differences in addictive and affective states [6].
This led them to reexamine the posibility of using kappa-opioids as analgesics, only in human females.
Researchers led by UCSF scientists are reporting that an experimental pain drug known as a kappa-opioid brings pain relief to female rats but not males, a finding that adds weight to a recent UCSF clinical finding, and highlights, they say, the need to evaluate drugs by gender.
Fields’ finding-that specific brain regions in male and female rats have opposite reactions to kappa-opioids, supporting clinical studies at UCSF that indicate kappa-opioids are more effective in women for clinically significant pain.
A clinical study led by UCSF professor Jon Levine, MD, PhD showed that, in women, a drug made up of a strong concentration of kappa-opioid has a strong and lasting analgesic effect.
In contrast, in men, the low dose actually increased pain; as the dose was increased, the heightened pain disappeared and a weak, short-lived analgesic effect set in.
The discovery, he says, demonstrates a clear biological difference in the way women and men respond to kappa-opioids.
Three years ago, the Fields team showed that treating the vlPAG neurons of male rats with a mu opioid brought about pain relief, but that subsequently adding kappa-opioid into the RVM markedly decreased the mu opioid’s analgesic effect.
Treating the vlPAG neurons of female rats with a mu opioid brought on the expected pain relief, but subsequently adding kappa-opioid into the RVM increased the mu opioid’s analgesic effect.
“In males, kappa-opioid is somehow inhibiting the actions of mu-opioid,” says Fields.
Kappa receptors are acting on opposite types of neurons in males and females. In males, kappas may be inhibiting the so-called “off” nerve cells in the RVM that normally tell the spinal cord to shut off pain signals. In females, kappa-opioids actually excite the off neurons, which would relieve pain.
In men, however:
The functionality of kappa- and delta-opioid receptors, might be less associated with relaxation and analgesic effects as kappa-OR often suppress activation of mu-opioid receptors, and delta-OR differ from mu-OR in its interaction with agonists and antagonists.

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
While the majority of sex difference findings related to KOR are from studies of the analgesic effects of kappa agonists, there is also emerging evidence of KOR-related sex differences in addictive and affective states [6].
This led them to reexamine the posibility of using kappa-opioids as analgesics, only in human females.
Researchers led by UCSF scientists are reporting that an experimental pain drug known as a kappa-opioid brings pain relief to female rats but not males, a finding that adds weight to a recent UCSF clinical finding, and highlights, they say, the need to evaluate drugs by gender.
Fields’ finding-that specific brain regions in male and female rats have opposite reactions to kappa-opioids, supporting clinical studies at UCSF that indicate kappa-opioids are more effective in women for clinically significant pain.
A clinical study led by UCSF professor Jon Levine, MD, PhD showed that, in women, a drug made up of a strong concentration of kappa-opioid has a strong and lasting analgesic effect.
In contrast, in men, the low dose actually increased pain; as the dose was increased, the heightened pain disappeared and a weak, short-lived analgesic effect set in.
The discovery, he says, demonstrates a clear biological difference in the way women and men respond to kappa-opioids.
Three years ago, the Fields team showed that treating the vlPAG neurons of male rats with a mu opioid brought about pain relief, but that subsequently adding kappa-opioid into the RVM markedly decreased the mu opioid’s analgesic effect.
Treating the vlPAG neurons of female rats with a mu opioid brought on the expected pain relief, but subsequently adding kappa-opioid into the RVM increased the mu opioid’s analgesic effect.
“In males, kappa-opioid is somehow inhibiting the actions of mu-opioid,” says Fields.
Kappa receptors are acting on opposite types of neurons in males and females. In males, kappas may be inhibiting the so-called “off” nerve cells in the RVM that normally tell the spinal cord to shut off pain signals. In females, kappa-opioids actually excite the off neurons, which would relieve pain.
In men, however:
The functionality of kappa- and delta-opioid receptors, might be less associated with relaxation and analgesic effects as kappa-OR often suppress activation of mu-opioid receptors, and delta-OR differ from mu-OR in its interaction with agonists and antagonists.

PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo
Opioid receptors may play critical roles in alcoholism and other addictions, addiction withdrawal, and depression and are considered pharmacological targets for treatment of these conditions. Sex differences have been demonstrated in mu (MOR) and delta ...
What these studies show is that mu- and kappa-opioid receptors work at cross-purposes in males, decreasing the rewarding effects of mu receptors.
However, the two receptors work synergistically in females; kappa activation increases the rewarding effects of mu receptors.
V. Estrogen is a natural painkiller that modulates opioid receptors to be more effective.
Estrogen is present in women at a level that is 10 to 15 times higher than the level present in men.
In 2011, researchers (Stenning et al) discovered that estrogen can modulate the density of opioid receptors.
Estrogen have also been found to induce mu-opioid receptor activation within the preoptic nucleus and posterodorsal medial amygdaloid nucleus.
High densities of estrogen receptors functionally related to endorphin receptors have been found within the hypothalamus, one of the primary sites of MOR. [mu-opioid receptors]
In addition, research on sex hormones indicates there is improved mu receptor binding in some brain regions in women after they receive estrogen, as measured by PET scans.
One of the conclusions forwarded from this research is that a decrease in estrogen would increase sensitivity to pain. Conversely, an increase in estrogen would promote analgesic effect by stimulating a bolus of pain-inhibiting transmitters.
The effects of estrogen modulation of pain receptors is further confirmed in a study by Dr. Kern Olson.
According to him:
Girls and boys react to pain in a similar fashion before puberty but differently after puberty; these differences, however, decrease as levels of sex hormones decrease as people age. (i.e., after menopause, when estrogen levels in women plummet)

Gender and the Pain Experience
Men and women experience pain differently. Find out more about the psychosocial and biological factors that influence our experience of pain.
Aside from their function in reproduction, sex hormones and their receptors that are widely distributed throughout the central nervous system have
demonstrated modulatory effects on the central opioid system to responses in pain.
Estrogen have also been found to induce mu-opioid receptor activation within the preoptic nucleus and posterodorsal medial amygdaloid nucleus.
High densities of estrogen receptors functionally related to endorphin receptors have been found within the hypothalamus, an area
with a high density of neuroendocrine and centrally projecting neurons.
(Toomey et al)
29 This effect can be blocked by the mu-opioid antagonist naltrexone,
29 which further demonstrates these hormoneopioid receptor interrelationships.
In addition, research on sex hormones indicates there is improved mu receptor binding in some brain regions in women after they receive estrogen, as measured by PET scans.
One of the conclusions forwarded from this research is that a decrease in estrogen would increase sensitivity to pain. Conversely, an increase in estrogen would promote analgesic effect by stimulating a bolus of pain-inhibiting transmitters.22

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
demonstrated modulatory effects on the central opioid system to responses in pain.
Estrogen have also been found to induce mu-opioid receptor activation within the preoptic nucleus and posterodorsal medial amygdaloid nucleus.
High densities of estrogen receptors functionally related to endorphin receptors have been found within the hypothalamus, an area
with a high density of neuroendocrine and centrally projecting neurons.
(Toomey et al)
29 This effect can be blocked by the mu-opioid antagonist naltrexone,
29 which further demonstrates these hormoneopioid receptor interrelationships.
In addition, research on sex hormones indicates there is improved mu receptor binding in some brain regions in women after they receive estrogen, as measured by PET scans.
One of the conclusions forwarded from this research is that a decrease in estrogen would increase sensitivity to pain. Conversely, an increase in estrogen would promote analgesic effect by stimulating a bolus of pain-inhibiting transmitters.22

Estrogen-Induced Alteration of μ-Opioid Receptor Immunoreactivity in the Medial Preoptic Nucleus and Medial Amygdala
The μ-opioid receptor (μ-OR), like most G-protein-coupled receptors, is rapidly internalized after agonist binding. Although opioid peptides induce internalization in vivo, there are no studies that demonstrate μ-OR internalization ...
VI. Opioid receptor activation is anti-sexual in men, pro-sexual in women.
According to the same study above:
Estrogens have also been found to induce mu-opioid receptor activation within the preoptic nucleus and posterodorsal medial amygdaloid nucleus (areas responsible for thermoregulation and sex behavior, respectively).
On a study on rats, an injection of mu-specific opioid drugs into the brains of male rats were seen to suppress male gonadal function. In other words, the rats were unable to maintain an erection after being administered these drugs, and mating behaviors decreased in response.
However, in female rats, administration of mu-specific drugs did not correlate with symptoms of sexual dysfunction.
On the contrary, when mu-opioids were administrated to female rats only in a group, unpaced mating increased. This nymphomania was increased the closer the rats got to estrus, corresponding with higher levels of estrogen. This was associated to higher rates of mu activation, which suggests a certain threshold of pain must be reached before sexual arousal happens.
This suggests that the pro-sexual effect of MOR activation on females will increase when estrogen levels are higher, such as during estrus, certain phases of the menstrual cycle, and the later terms of pregnancy.
The researchers reported that with lower levels of estrogen, progesterone was pronociceptive.
Thresholds were decreased, and pain intensities increased during the midluteal phase when progesterone levels were relatively higher than estrogen levels.
[19] This system is also thought to be important in mediating complex social behaviors involved in the formation of stable, emotionally committed relationships. Social attachment was demonstrated to be mediated by the opioid system through experiments administering morphine and naltrexone, an opioid agonist and antagonist, to juvenile guinea pigs. The agonist decreased the preference of the juvenile to be near the mother and reduced distress vocalization whereas the antagonist had the opposite effects. Experiments were corroborated in dogs, chicks, and rats confirming the evolutionary importance of opioid signaling in these behaviors.
[18] Researchers have also found that systemic naltrexone treatment of female prairie voles during initial exposure to a male reduced subsequent mating bouts and nonsexual socialization with this familiar partner, when a choice test including a novel male was performed afterwards. This points to a role for opioid receptors in mating behaviors.

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
Stenning et al studied the pain response across the menstrual cycle phases using a cold pressure test. In this study, a demonstration of variations in pain perception that correlate with the fluctuating concentration ratios of estrogen and progesteronewas conducted. The researchers reported that with lower levels of estrogen, progesterone was pronociceptive.
thresholds were decreased, and pain intensities increased during the midluteal phase when progesterone levels were relatively higher than estrogen levels.
Briefly, opioid receptor agonists injected directly into the MPOA inhibited or delayed masculine copulatory activity in rats. Indeed, when injected into this structure, opioid agonists markedly impaired penile erection
Opioids frequently cause low FT levels in men, but there is no relationship between abnormal hormone levels and symptoms of sexual dysfunction. Therefore, all men should be screened for low FT levels. Women on opioids had lower FT levels, but this did not correlate with sexual dysfunction symptoms.

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
[18] Researchers have also found that systemic naltrexone treatment of female prairie voles during initial exposure to a male reduced subsequent mating bouts and nonsexual socialization with this familiar partner, when a choice test including a novel male was performed afterwards. This points to a role for opioid receptors in mating behaviors.

Robust age, but limited sex, differences in mu-opioid receptors in the rat brain: relevance for reward and drug-seeking behaviors in juveniles
In the brain, the µ-opioid receptor (MOR) is involved in reward-seeking behaviors and plays a pivotal role in the mediation of opioid use disorders. Furthermore, reward-seeking behaviors and susceptibility to opioid addiction are particularly ...
Stenning et al studied the pain response across the menstrual cycle phases using a cold pressure test. In this study, a demonstration of variations in pain perception that correlate with the fluctuating concentration ratios of estrogen and progesteronewas conducted. The researchers reported that with lower levels of estrogen, progesterone was pronociceptive.
thresholds were decreased, and pain intensities increased during the midluteal phase when progesterone levels were relatively higher than estrogen levels.
Briefly, opioid receptor agonists injected directly into the MPOA inhibited or delayed masculine copulatory activity in rats. Indeed, when injected into this structure, opioid agonists markedly impaired penile erection
Opioids frequently cause low FT levels in men, but there is no relationship between abnormal hormone levels and symptoms of sexual dysfunction. Therefore, all men should be screened for low FT levels. Women on opioids had lower FT levels, but this did not correlate with sexual dysfunction symptoms.

Opioid analgesics suppress male gonadal function, but opioid use in men and women does not correlate with symptoms of sexual dysfunction
Opioid analgesia impairs gonadal function in men and women, but the correlation with symptoms and hormonal measurements of hypogonadism is not well established.To determine the frequency of impaired gonadal function in men and women using opioids for ...
This pro-sexual phenomenon can be observed in human females as well. Estrogen serves to increase the feelings of sexual arousal derived from pain.
This effect can be seen in the “birthgasm.” During pregnancy, the estrogen levels in a women increase the closer she gets to giving birth. This effect is seen during pregnancy and may account for pregnancy-induced increases in tolerance to nociception. This may have evolved so that a woman could better tolerate the “pain” of childbirth. As a result, a woman’s estrogen levels are necessarily highest just before and during the act of giving birth. Estrogen and MOR activation working in tandem have often resulted in women having orgasms during childbirth:
When a woman feels the contractions of an orgasm and/or extreme moments of pleasure right at the moment of delivering her baby, this may be called an “orgasmic birth.” You may feel tremendous pressure and sensation in the vagina as your baby's birth approaches, then a powerful, pleasurable release that's both physical and orgasmic.
This phenomenon is common enough that the term of “birthgasm” was coined.
5. Acupuncture
Acupuncture was a saving grace. It helps more than anything else I tried.
- Molly Qerim, a foid
***
Acupuncture is a form of alternate medicine in which thin needles are inserted into the body. The practice of acupuncture is considered a pseudoscience because the theories and practices of traditional Chinese medicine -- based on the concepts of qu, meridians, and acupuncture points, life force energy -- are not amenable to modern scientific knowledge, and it has been characterized as quackery.
Many scientific reviews have found that acupuncture is ineffective for a wide range of conditions.
Some research results suggest that it can alleviate some forms of pain, though the majority of research suggests that its apparent effects are not caused by the treatment itself. Many acupunctures attribute pain relief to the release of endorphins when needles penetrate.

Acupuncture - Wikipedia
In other words, there is a prevailing theory that the way acupuncture “works” is by causing pain that causes the body to go into pain control overdrive, releasing endorphins and creating a state of well-being.
Unsurprisingly, the great majority of proponents of acupuncture are female. By some estimates, the ratio of women to men who use acupuncture regularly is 5 to 1. (This especially applies to East Asian women, who are on average more masochistic and exogamic than women of other races.) Discounting the placebo effect, the logical explanation is that acupuncture works for women, but not men.

Gender Differences in Traditional Chinese Medicine Use among Adults in Taiwan
The increasing use of complementary, alternative medicine (CAM) and traditional Chinese medicine (TCM) has attracted attention. We report on the gender difference in TCM use among the general population in Taiwan in a population-based, cross-sectional ...
To see why this is, let’s examine the differences of male and female brains’ reactions in response to acupuncture.
In males:
The needle is inserted.
Pain is sensed by the nerves.
A small number of rewarding mu receptors activate, and a large number of punishing kappa receptors activate.
Mu receptors try to “reward” for the pain. However, the kappa receptors lessen this effect.
Male feels pain and aversion. He begins to associate acupuncture with negative feelings of dysphoria.
The man’s sexual desire decreases, and erection becomes impossible.
In females:
The needle is inserted.
Pain is sensed by the nerves.
A large number of rewarding mu receptors activate, and a small number of synergistically rewarding kappa receptors activate.
Mu rewards the woman for the pain. Kappa helps this effect, transforming pain into pleasure.
Estrogen modulates the pain response, causing sexual arousal.
Female feels euphoria and sexual arousal. She begins to associate acupuncture with positive feelings of euphoria.
The woman’s sexual desire increases; her desire for unpaced mating goes up.
It’s not difficult to see, then, why women are the primary consumers of acupuncture.
***
A famous example of acupuncture working as advertised proves my point further.
In the early 20th century, surgeons performed open heart surgery on a 15-year-old girl in China without using anesthesia. The only measures taken to alleviate the pain of the surgery was acupuncture. Nevertheless, it is said that girl remained calm and immobile during the operation, which was a great success. To this day, this is held up as one of the primary pieces evidence of evidence in support of the efficacy of acupuncture.
However, notice that it was a girl who received the surgery, and not a boy. A male would scarcely have been able to endure the pain of the operation. However, it would have been no difficulty at all for a female.
The patient in this stamp was a 15 year old girl with congenital ventricular septal defect. The Chinese made disc oxygenator for total cardiopulmonary bypass can be seen on the right. The anesthetist who performed the acupuncture-two fine needles inserted in both wrists and a further two in the anterior chest wall at both subclavicular areas-was at the patient's head; he was a practitioner of Chinese traditional medicine. The surgeon on the patient's left was Professor Yi-shan Wang, my schoolmate in St John's University School of Medicine, Shanghhai, China, and the surgeon on the patient's right was Dr Chun-xiu Yeh, my classmate in the same school.
Open heart surgery under acupuncture anaesthesia is depicted on this 8 cent Chinese stamp issued in 1975 as part of a set of four stamps to commemorate the successful integration of traditional Chinese medicine and modern Western medicine in the treatment of various diseases.
The four Chinese characters at right lower corner of the stamp stand for acupuncture anaesthesia. The other three stamps in the set feature such surgical feats as replantation of severed limbs, application of small soft splints for fractures, and cataract surgery.
The patient in this stamp was a 15 year old girl with congenital ventricular septal defect. The Chinese made disc oxygenator for total cardiopulmonary bypass can be seen on the right. The anesthetist who performed the acupuncture-two fine needles inserted in both wrists and a further two in the anterior chest wall at both subclavicular areas-was at the patient's head; he was a practitioner of Chinese traditional medicine. The surgeon on the patient's left was Professor Yi-shan Wang, my schoolmate in St John's University School of Medicine, Shanghhai, China, and the surgeon on the patient's right was Dr Chun-xiu Yeh, my classmate in the same school.
The four Chinese characters at right lower corner of the stamp stand for acupuncture anaesthesia. The other three stamps in the set feature such surgical feats as replantation of severed limbs, application of small soft splints for fractures, and cataract surgery.
The patient in this stamp was a 15 year old girl with congenital ventricular septal defect. The Chinese made disc oxygenator for total cardiopulmonary bypass can be seen on the right. The anesthetist who performed the acupuncture-two fine needles inserted in both wrists and a further two in the anterior chest wall at both subclavicular areas-was at the patient's head; he was a practitioner of Chinese traditional medicine. The surgeon on the patient's left was Professor Yi-shan Wang, my schoolmate in St John's University School of Medicine, Shanghhai, China, and the surgeon on the patient's right was Dr Chun-xiu Yeh, my classmate in the same school.
6. Conclusion
Proposed Changes to Society:
1. Medication.
Opioids and anesthetics are inherently dangerous to administer to women, since there is a greater chance of overdose and addiction. (See III.A)
As a result, it would be beneficial for their safety if anesthetics were not administered during female patients’ surgeries.
Anesthesia can be replaced with acupuncture for female patients, which, as shown above (5), has comparable efficacy and far less risk.
This will also increase supply and decrease demand of anesthesia medication, leading to a decrease in the cost of expensive anesthesia procedures.

Gender and the Pain Experience
Men and women experience pain differently. Find out more about the psychosocial and biological factors that influence our experience of pain.
2. Abortion.
Many current proponents of abortion justify the legality of early-term abortion by the logic that the baby does not yet feel pain.
Following this logic, it would seem that crimes are more serious the more pain is caused.
But women essentially feel no physical pain, as shown above.
To maintain legal consistency, it follows that either abortion must be classed as murder, or crimes that cause bodily injury (battery and domestic assault) to women should not be punished as harshly as those which cause bodily injury to men.
3. Scientific Studies.
German scientists and physicians made great medical advances in the 1940s, due to their progressive genetic research and innovative surgical experimentation, particularly in the area of twins. This was a time of medical enlightenment, and many of the concepts they discovered are still used today. Various legislative considerations, however, prevent us from conducting similar studies today.
However, since our knowledge of the nature of human suffering was then incomplete and now we are more informed, some of these restrictions can now be loosened on experimentation on female subjects.
@ShadowTheEdgehog
@Mainländer
Attachments
Last edited: