InMemoriam
Celiacel
★★★★★
- Joined
- Feb 19, 2022
- Posts
- 8,732
Three-dimensional analysis of modeled facial aging and sexual dimorphism from juvenile to elderly age
Show authorsScientific Reports volume 12, Article number: 21821 (2022) Cite this article
- 936 Accesses
- 22 Altmetric
- Metricsdetails
Abstract
A detailed understanding of craniofacial ontogenetic development is important in a variety of scientific disciplines dealing with facial reconstruction, forensic identification, ageing prediction, and monitoring of pathological growth, including the effect of therapy. The main goals of this study were (1) the construction of the facial aging model using local polynomial regression fitting separately for both sexes, (2) evaluation of the aging effect not only on facial form as a whole but also on dimensions important for clinical practice, and (3) monitoring of the development of shape facial sexual dimorphism. Our study was based on the form and shape analysis of three-dimensional facial surface models of 456 individuals aged 14–83 years. The facial models were obtained using a structured light-based optical scanner and divided (for some analyses) into four age categories (juveniles, young adults, middle adults, and elderly adults). The methodology was based on geometric and classic morphometrics including multivariate statistics. Aging in both sexes shared common traits such as more pronounced facial roundness reducing facial convexity, sagging soft tissue, smaller visible areas of the eyes, greater nose, and thinner lips. In contrast to female faces, male faces increase in size until almost 30 years of age. After the age of 70, male facial size not only stagnates, like in females, but actually decreases slightly. Sexual dimorphic traits tended to diminish in the frontal and orbitonasal areas and increase in the gonial area.Introduction
Accurate and complex evaluation of facial morphology is dependent on the understanding of ontogenetic facial development, including variability, sexual dimorphism, facial expression and pathological deformity. The knowledge of the age- and sex-related qualitative and quantitative characteristics provides useful information both in medicine and forensic sciences; recently it has been useful in connection with the perception of attractiveness, in ethology and the morphological divergence of the human face among populations1,2.Medical reference facial data are necessary for maxillofacial surgery, plastic surgery, genetics and orthodontists. With regard to pre- and post-operative treatment, the comparisons of facial differences between patients with craniofacial anomalies or syndromes with those fulfilling normative values, as well as comparisons between different age and sex groups, are important in deciding on an appropriate therapeutic course3,4.
Figure 1
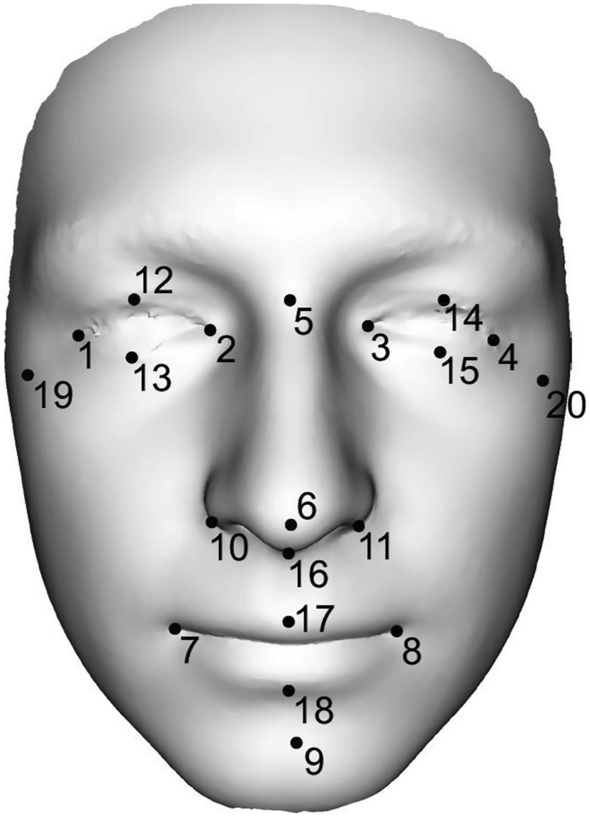
Representation of 20 landmarks on the 3D facial scan. The following dimensions were measured between the individual landmarks: ocular width (1–2, 3–4), ocular height (12–13,14–15), intercanthal width (2–3), biocular width (1–4), distances between exocanthion–nasion (1–5, 4–5), nasal length (5–6), nasal width (10–11), nasal depth (6–16), philtrum height (16–17), nasal height (5–16), dimension between pronasale–pogonion (6–9), mouth width (7–8), mouth height (17–18), facial height (5–9), lower face height (9–16) and facial width (19–20).
Results
The results are divided into three parts, with the first dealing with the complex geometry of the face. Detailed morphological age-related facial changes were evaluated using aging trajectories in the space of centroid size and extracted synthesized facial models (for 15, 20, 30, 40, 50, 60, 70 and 80 years of ages) and using a superimposition method of average male and female faces and colour-coded maps. The second part is devoted to the development of sexual dimorphism of the facial shape; the third part to classic morphometry. We evaluated how facial dimensions change from the youngest to the oldest age categories. The statistical significance of sexual dimorphic difference was also analyzed.Modeled facial development from juvenile to elderly age
Modeled facial development from juvenile to elderly age was visualized using the Figs. 2 and 3. In general, it can be stated that with increasing age, the faces of both sexes became larger and wider until the age of 70 (Figs. 3 and 4). A woman’s face changes very little by the age of 30, while a man’s face increases the most at that age. From 30 to 60 years of age, the faces of both sexes increase in size and widen slightly. Unlike women’s faces, men’s faces after the age of 60 shrink slightly. Aging after the age of thirty is manifested not only by the widening of the face, but also by the sagging of soft tissue and increased visibility of skin folds (Fig. 4).Figure 2
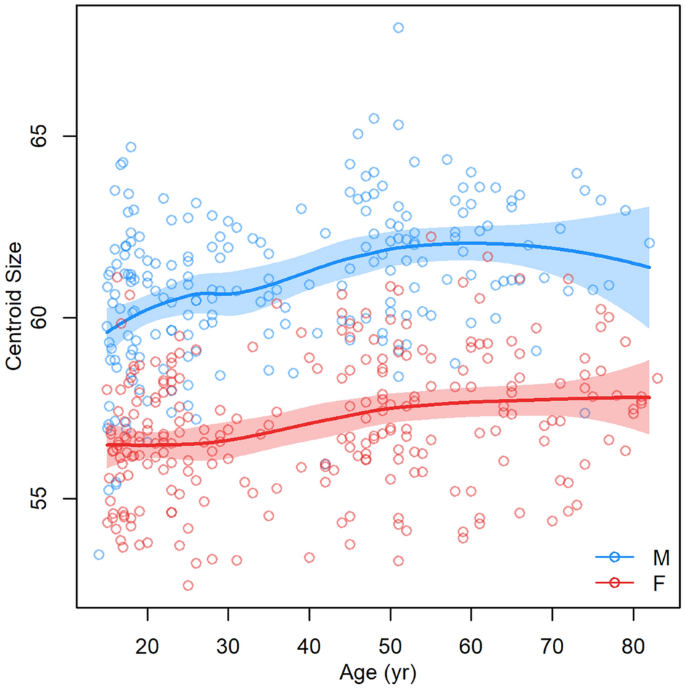
Dependence of facial centroid size on increasing age (the function was constructed separately for females and males as curves with a 95% confidence region).
Figure 3
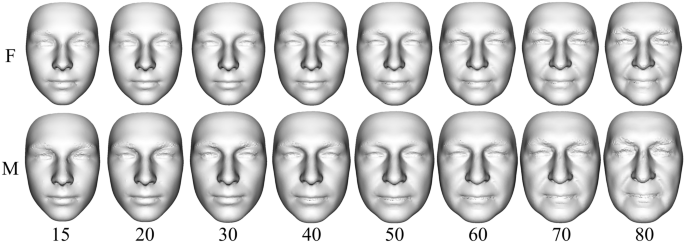
Predicted synthesized facial models for 15, 20, 30, 40, 50, 60, 70 and 80 years of ages created using 200 PC contributions to the mean form (F—females, M—males).
Figure 4

Colour-coded maps and shell distance significance maps describing average facial form differences between 0 and I, I and II, II and III age categories in females (upper row) and males (lower row). The most protrusive parts of the average faces are represented in red, whereas those that are situated deeper are coloured blue. The statistical significance of form differences was analysed per vertex and coded in shades of blue (significant differences) or grey (no significant differences) on the superimposed average faces.
Figure 5
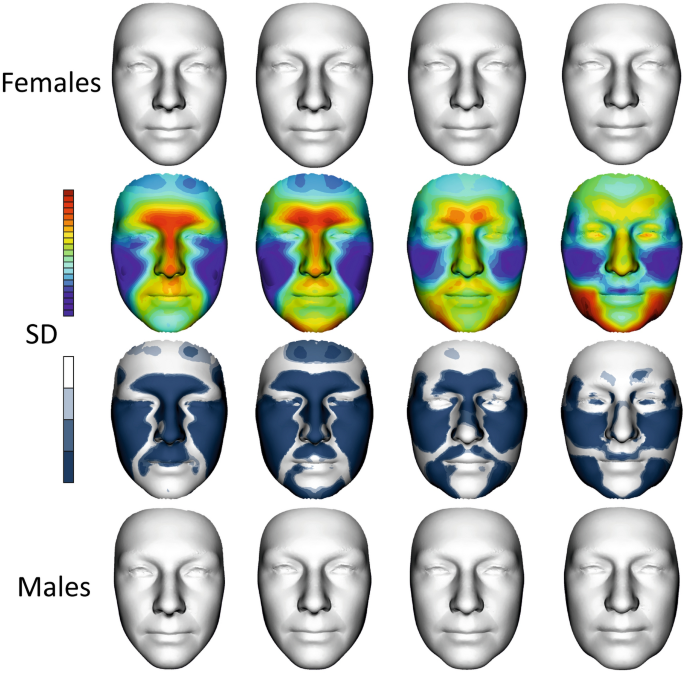
Development of sexual dimorphism of facial shape. The first row shows female average faces in all age categories, i.e., 0, I, II and III. The second row is a superimposition of the mean facial shapes of males and females in all age categories. The colour maps show the relative reciprocal locally inward/outward positions of the mean male and female facial shapes. Red denotes that a particular area of the male face was located in front of the female face after superimposition, while blue indicates the converse condition. The third row shows shell distance significance of sexual dimorphic maps in each age category. The last row shows average male faces in all age categories. (SD—shell distance).
Generally, the average male models tended to have more protruded lower parts of the forehead, eyebrow ridges, nose, upper lip and area of the philtrum compared to the female average; however, these differences were substantially reduced as they approached category III. Conversely, the males had deeper eye regions in the first age category, which were simultaneously reduced to the deeper female eye position in the last age category.
When we dealt with the juvenile age category, the sexual dimorphism of the upper part of the frontal bone and primarily area of the mandible was less apparent than in young adulthood. Sexual dimorphism in young adulthood was the largest and significant in all the monitored areas of the face. In the first and second age categories, the nasal length, eyebrow ridges, and upper lip were more protruded in males, while the superior part of the forehead and the cheeks were more protruded in females. Protruded cheeks and a more rounded face in females seemed to be the most stable sexual dimorphic features during aging (see maps of significance of all investigated categories).
Male protrusion of the chin was typical and largest in the second age category from 20 to 40 years. Chin prominence decreased with aging, but the width of the male mandible region increased. This widening of the lower third of the male face was related to the loss of the jawline, which changed from fluent to more fragmented, especially in the last age category.
When we look at the significance maps of facial shapes from juvenile to elderly age (Fig. 5, 3rd row of faces), we can see that sexual dimorphism was reduced with increasing age in the forehead, nose, upper lip, and chin; conversely, it was the most stable in the cheek area. Sexual dimorphism of the mandible region started to develop after 20 years of age in the chin area. The lateral parts of the lower face had the most sexual dimorphic difference after 60 years of age, when chin prominence is diminished. When we evaluated the face as a whole, sexual dimorphism was the smallest in the last age category.
can't wait to get 10 fold uglier
Last edited:





